Current Research
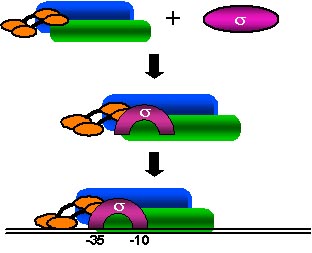
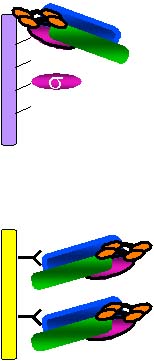
Work in this laboratory focuses on prokaryotic and eukaryotic RNA polymerases (RNAPs) and transcription machinery and their roles in RNA synthesis and its regulation. We study the structure and function of the initiation factor, E. coli RNAP sigma70 subunit, by a concerted use of protein and physical chemistry, monoclonal antibodies (MAbs), molecular genetics, computer-based sequence and structure analysis, and biochemistry.
We have overproduced and purified all seven known E. coli sigma factors and have made MAbs to them that can be used in measuring their levels in the cell under various growth conditions, inhibiting them, and immunoaffinity purifying them. We have developed a powerful new method, using histidine-tagged RNAP subunit fragments, to map epitopes of our various MAbs easily and quickly. We have recently utilized a variation of this method, employing "ordered fragment ladder far-Western blotting", to map interaction domains and have identified a major binding site for sigma70 within the region of amino acids 260-309 of the beta prime subunit of core RNAP.
We are also determining if the other E.coli sigmas bind in the same manner as sigma 70. Understanding these interactions in detail will allow us to better understand the complex but central mechanism of transcription and to design potentially important new antibiotics that work by disrupting transcription in pathogenic bacteria.
We are utilizing special "polyol-responsive" MAbs that we have discovered to immunoaffinity purify RNAP II from human and yeast cells. We are also studying the interactions of human RNAP II with the basal transcription factors, TBP, Rap30, and TFIIB.